In a significant development for the field of neurotechnology, Elon Musk’s ambitious startup, Neuralink, has announced the successful implantation of its brain-computer interface device in a human patient. The procedure, a crucial step in the company’s clinical trials, was carried out at a hospital located in the Miami area, marking a pivotal moment for the company and the broader scientific community.
This event represents the culmination of years of research and development by Neuralink, a company founded by Musk with the audacious goal of creating a direct interface between the human brain and computers. The stated purpose of this technology is multifaceted, initially focusing on assisting individuals with severe neurological conditions. By enabling direct neural communication with external devices, Neuralink aims to restore lost functions and improve the quality of life for patients dealing with paralysis, blindness, deafness, and other debilitating disorders.
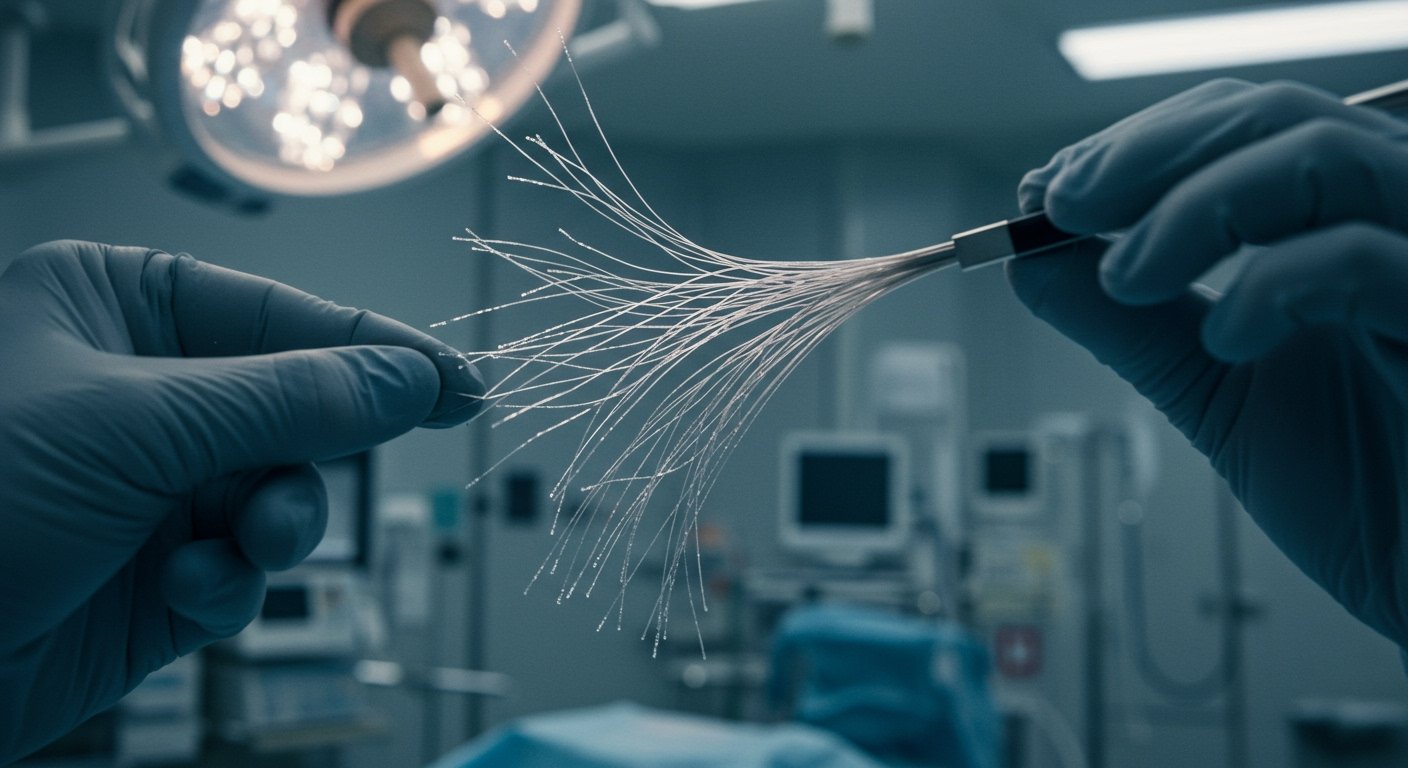
The implant itself, often referred to as Elon Musk’s brain chip, is a highly sophisticated device designed to be surgically placed within the brain. Its core technology involves incredibly fine threads, thinner than a human hair, that are intended to be inserted into the brain tissue to read neural activity. This information is then transmitted wirelessly to an external receiver, which can interpret the brain signals and potentially translate them into commands for controlling computers or prosthetic limbs.
The specific details surrounding the patient and the procedure at the Miami-area hospital remain limited, in line with typical clinical trial confidentiality. However, the confirmation that the device was successfully put in a patient at a facility within the Miami area validates the company’s progress from preclinical studies to human application. This marks the first documented instance of Neuralink’s device being implanted in a patient, moving the technology from theoretical potential and animal testing into the realm of human clinical investigation.
The selection of a hospital in the Miami area for this groundbreaking procedure underscores the presence of advanced medical facilities and expertise in the region capable of performing complex neurosurgical interventions required for such implants. While the specific institution has not been widely disclosed, its location in the Miami area is a key geographic detail associated with this historic event.
Neuralink’s journey to human trials has been a subject of intense scrutiny and anticipation. The company received approval from the U.S. Food and Drug Administration (FDA) to conduct its first-in-human clinical trial, known as the PRIME (Precise Robotically Implanted Brain-Computer Interface) study. This approval was a critical regulatory hurdle, allowing Neuralink to proceed with testing the safety and efficacy of its fully implantable, wireless brain-computer interface for individuals with paralysis.
The PRIME study’s primary objective is to evaluate the safety of the implant and the surgical robot, as well as to assess the initial functionality of the device. For individuals with significant paralysis, the ability to control external technology – such as computer cursors or keyboards – using only their thoughts could be transformative, offering new avenues for communication and independence.
While the recent implantation in the Miami area is a significant technical achievement, it is merely the first step in a potentially long and complex clinical trial process. The coming months will involve monitoring the patient, evaluating the performance of the implant, and assessing any potential side effects. Success in this initial trial phase will be crucial for Neuralink to gain further regulatory approvals and potentially expand its studies to a larger patient population.
The field of brain-computer interfaces is not new, with other research groups and companies having developed and implanted devices for years. However, Neuralink’s approach, particularly its high-density electrode array and wireless design, along with the high profile of its founder, Elon Musk, has brought unprecedented public attention to the technology. The stated long-term vision extends beyond medical applications, with Musk envisioning a future where such implants could enhance human capabilities or even enable a form of symbiotic relationship with artificial intelligence, though these goals remain highly speculative and far off.
Ethical considerations surrounding brain implants are substantial. Issues such as data privacy, security of neural information, potential for misuse, and equitable access to the technology are subjects of ongoing debate among scientists, ethicists, and policymakers. As Neuralink progresses, these ethical frameworks will need careful consideration to ensure the technology is developed and deployed responsibly.
The successful implantation at the Miami-area hospital represents a tangible stride in bridging the gap between human cognition and digital technology. For the patient involved, it offers potential hope for regaining some control over their interaction with the world. For Neuralink and Elon Musk, it is a validation of their technological approach and the start of gathering critical human data. For the broader scientific and medical communities, it signifies the accelerating pace of innovation in neurotechnology.
While the path forward involves rigorous testing, evaluation, and addressing complex challenges, the fact that Elon Musk’s Neuralink brain chip has now been put in a patient at a hospital in the Miami area firmly establishes this moment as a notable chapter in the history of brain-computer interfaces and their potential to redefine human capability and address severe neurological impairments.